Indications and Usage
LACRISERT® (hydroxypropyl cellulose ophthalmic insert) is indicated in patients with moderate to severe dry eye syndromes, including keratoconjunctivitis sicca. LACRISERT® is indicated especially in patients who remain symptomatic after an adequate trial of therapy with artificial tear solutions. LACRISERT® is also indicated for patients with exposure keratitis, decreased corneal sensitivity, and recurrent corneal erosions.
Important Safety Information
- LACRISERT® (hydroxypropyl cellulose ophthalmic insert) is contraindicated in patients who are hypersensitive to hydroxypropyl cellulose.
- Instructions for inserting and removing LACRISERT® should be carefully followed.
- If improperly placed, LACRISERT® may result in corneal abrasion. Because LACRISERT® may cause transient blurred vision, patients should be instructed to exercise caution when driving or operating machinery.
- The following adverse reactions have been reported, but were in most instances mild and temporary: transient blurring of vision, ocular discomfort or irritation, matting or stickiness of eyelashes, photophobia, hypersensitivity, eyelid edema, and hyperemia.
To report suspected adverse reactions, contact Bausch & Lomb Incorporated at 1-800-321-4576 or FDA at 1-800-FDA-1088 or FDA.gov/medwatch.
Click here for full Prescribing Information
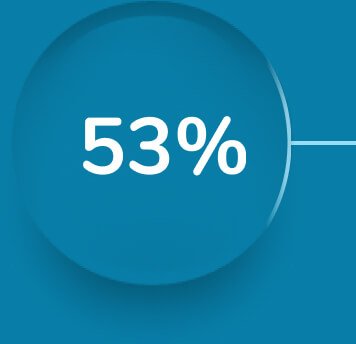
 Sustained relief
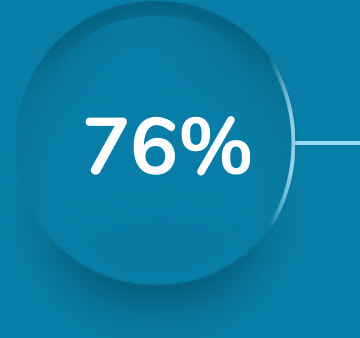
Sustained relief