
*In most patients, one LACRISERT® placed into each eye, once daily, is usually effective in providing all-day symptom relief.
Some patients may require twice daily use for optimal results.

Some patients may require twice daily use for optimal results.
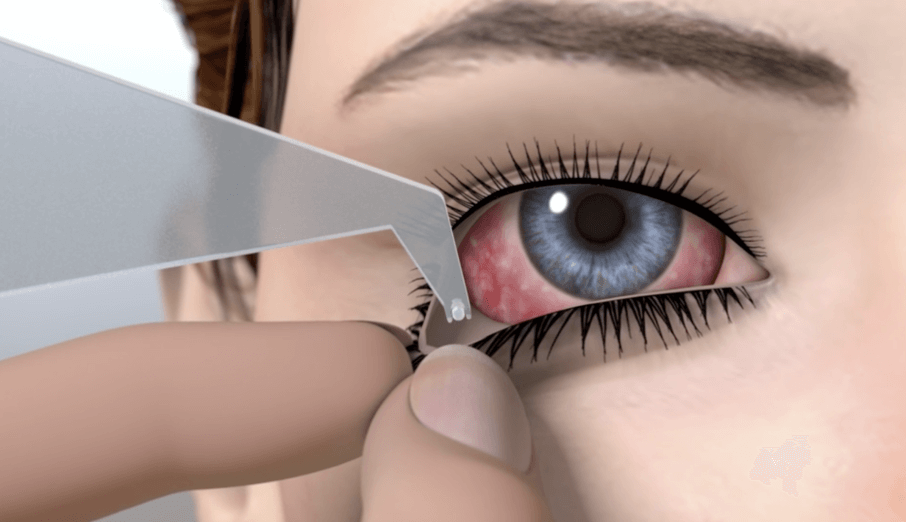
The LACRISERT® insert softens within 1 hour and dissolves in 14 to 18 hours.†
†Based on pre-clinical data.
Our featured eye doctors discuss topics like moderate to severe dry eye prevalence in their practices, why they choose LACRISERT®, and what patient types may benefit from treatment.
Paul Karpecki, OD
Koffler Vision Group
Doug Devries, OD
Eyecare Associates of Nevada
Jack Schaeffer, OD
Schaeffer Eye Center
LACRISERT® (hydroxypropyl cellulose ophthalmic insert) is indicated in patients with moderate to severe dry eye syndromes, including keratoconjunctivitis sicca. LACRISERT® is indicated especially in patients who remain symptomatic after an adequate trial of therapy with artificial tear solutions. LACRISERT® is also indicated for patients with exposure keratitis, decreased corneal sensitivity, and recurrent corneal erosions.
To report suspected adverse reactions, contact Bausch & Lomb Incorporated at 1-800-321-4576 or FDA at 1-800-FDA-1088 or FDA.gov/medwatch.
Click here for full Prescribing Information
LACRISERT® (hydroxypropyl cellulose ophthalmic insert) is indicated in patients with moderate to severe dry eye syndromes, including keratoconjunctivitis sicca. LACRISERT® is indicated especially in patients who remain symptomatic after an adequate trial of therapy with artificial tear solutions.
LACRISERT® (hydroxypropyl cellulose ophthalmic insert) is contraindicated in patients who are hypersensitive to hydroxypropyl cellulose.