Presentation
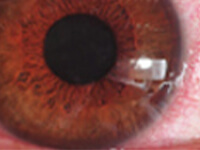
A 62-year-old Asian female presented with chief complaints of blurry vision, mild burning, redness, watery eyes, and slight pain. She reported that her symptoms were worse on windy days and she regularly woke up at night to put drops in. She had been diagnosed with severe dry eye, and her medical history was significant for Sjögren’s syndrome (8 years), rheumatoid arthritis, and mixed connective tissue disorder.
The patient had previously been given moisture retention goggles and punctal plugs to treat her dry eyes. At the time of presentation, her ocular medications included topical cyclosporine 0.05%, a dual acting mast cell stabilizer/antihistamine agent as needed, topical corticosteroid, and preservative-free artificial tears to be taken hourly in both eyes. She was also taking systemic medications, including the anti-inflammatories naproxen, hydroxychloroquine, and prednisone, plus oral pilocarpine for her dry mouth, and medications for hypertension (atenolol and amlodipine) and allergy (fluticasone and loratadine).