Presentation
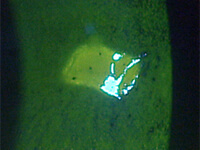
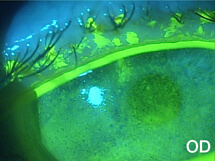
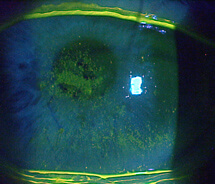
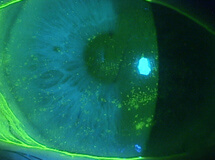
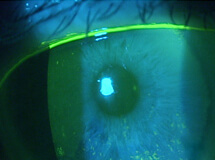
A 78-year-old female was referred to my clinic for evaluation of corneal abrasions and mucopurulent discharge that had persisted for at least 3 to 4 weeks. Her chief complaint was that her eyes felt like they were “full of sand,” and she was unable to see well. She also described both eyes as burning, painful, itchy, red, and sensitive to light. She was an avid reader but had not been able to read for over a year. At the time of her referral, she was using artificial tears every 2 hours and erythromycin ointment BID.
The patient had a longstanding history of moderate to severe dry eye, for which she had been offered and tried numerous treatments over the years, including artificial tears, corticosteroids, topical cyclosporine, punctal occlusion, and autologous serum. She had had cataract surgery about 8 years prior, and she stated that she had been given antiviral therapy for unilateral “red eye” about 15 years ago. Her medical history was significant for diabetes.
The patient’s symptoms of foreign body sensation, blurry vision, eye pain, and photophobia appear to be consistent with corneal abrasion, but she lacked a definite history of ocular injury, the most common cause of corneal abrasion.1 The discharge she reported caused me to suspect an infection.
- Fraser S. Corneal abrasion. Clin Ophthalmol. 2010;4:387-90.